Continuous Purification: MCSGP with AutoPeak® Technology
Batch v. MCSGP
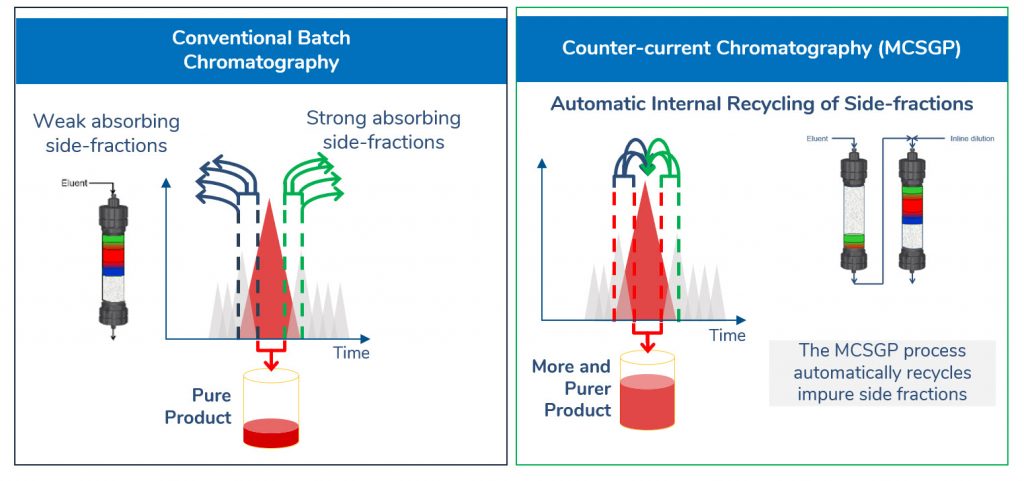 In batch chromatography of peptides and oligos, impure side fractions often have to be discarded to reach a certain product purity. With our patented MCSGP approach using AutoPeak technology, impure product-containing side fractions are internally (automatically) recycled in a periodic closed-loop process while pure product is continuously extracted. Thereby, little product is lost, and the yield of pure product is maximized without any accumulation of impurities while retaining target purity.
In batch chromatography of peptides and oligos, impure side fractions often have to be discarded to reach a certain product purity. With our patented MCSGP approach using AutoPeak technology, impure product-containing side fractions are internally (automatically) recycled in a periodic closed-loop process while pure product is continuously extracted. Thereby, little product is lost, and the yield of pure product is maximized without any accumulation of impurities while retaining target purity.
High yield at target purity
MCSGP economic benefits
Multicolumn Countercurrent Solvent Gradient Purification process (MCSGP) is a continuous process operated by Contichrom lab-scale Contichrom CUBE and Contichrom TWIN pilot/process scale equipment using AutoPeak® technology. It provides significant economic benefits compared to single-column batch chromatography operated at low, medium, and high pressure. The economic benefits are derived by achieving significantly higher yield at target purity, lower buffer/solvent consumption, and higher productivity. Regardless of the products or applications, MCSGP with AutoPeak technology will provide economic benefits. The more complex the separation, the bigger the savings.
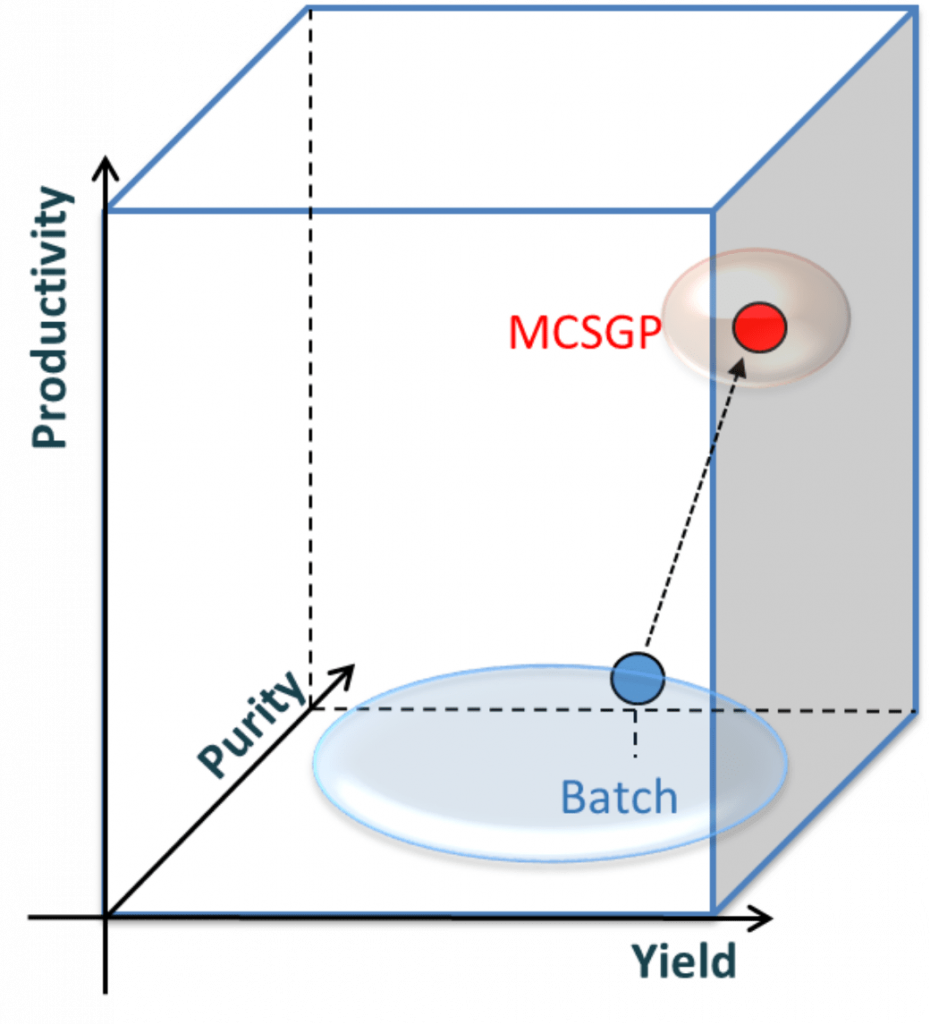
Figure: Yield-purity-productivity relationship of MCSGP with AutoPeak technology versus conventional batch chromatography. Single-column batch processes (blue) operate on a low productivity level without the potential for productivity gains when high yield and purity are required. MCSGP (red) operates at an optimum in 3D space, with high yield and purity at increased productivity.
Application of MCSGP for peptide and oligo purification
High performance liquid chromatography employing MCSGP with AutoPeak technology provides a novel purification process for peptides or oligos produced by chemical synthesis. MCSGP offers a step-change in efficiency compared to batch HPLC processing. With MCSGP, two identical reverse-phase columns are operated under high pressures in countercurrent mode with internal recycling of impurity-containing side fractions, extracting continuously pure product and discarding impurities without significant product loss. Peptides can be purified at preparative/production scale with a significantly higher yield without compromising target purity. The process allows up to tenfold higher productivity with potentially 75% lower solvent consumption, providing an overall attractive economical production scenario and pushing the boundary of economic synthesis of long peptides.
Application of MCSGP for omega-3 EPA purification
MCSGP with AutoPeak technology can purify complex biological mixtures, such as omega-3 eicosapentaenoic acid ethyl ester (EPA-EE) to a high target purity in a single purification step. Generic fish oil feed with a feed purity of 74% EPA-EE was subjected to an MCSGP process design to achieve a target purity of >97% EPA-EE. The process operated under high pressures in countercurrent mode with internal recycling of impurity-containing side fractions with docosahexaenoic acid ethyl ester (DHA-EE) as the main impurity, extracting continuously pure EPA-EE and discarding impurities without significant product loss. Five cycles of MCSGP with AutoPeak technology achieved significantly improved yield at target purity (+ 250%), productivity throughput (+ 590%), and reduced solvent consumption by 75% compared to batch HPLC.
Process principle and design
The MCSGP process with AutoPeak technology is the only known ternary continuous separation process that can unlock the yield-purity trade-off dilemma by providing high yield at target purity, thereby enhancing productivity.
The process eliminates the need for re-chromatography and the associated time and resources for sampling and testing. The process principle of our patented MCSGP with AutoPeak technology is intuitive, straightforward, and allows a high degree of automation. A dedicated software wizard in the accompanying ChromIQ software allows for easy batch-to-continuous process design, transfer, and optimization.
YMC offers customized services and expertise to companies and academics who wish to quickly advance their projects or test MCSGP with AutoPeak capabilities before investing in a system.
MCSGP with AutoPeak technology is used for:
- Small molecules from fermentation or natural extractions (Stevia, flavonoids, fish oil)
- Blood plasma products
- Recombinant proteins from microbial production
- Complex organic synthesis products
MCSGP resources

Continuous purification of therapeutics—removing critical barriers in manufacturing by MCSGP

Purification of a Therapeutic Oligonucleotide Using Twin-Column Chromatography
Chiral Prep Chromatography Utilizing Twin-Column Technology
Application Search
Use our extensive Applications Search page to mine a dynamically growing collection of over 350 YMC-produced application notes.
The data can be searched by any combination of several variables, including sample classification, compound name (including partial names), and column parameters.
