Peptides
Peptide chemistry advances are driving increased use
Peptide-based therapeutics are noted for being highly selective and efficacious yet relatively safe and well tolerated by the body. While the use of peptides as treatments has been around for decades, recent advances in the science of physical and chemical stability of peptides have accelerated interest. To date, more than 140 clinical studies—especially in the areas of metabolic diseases and oncology—have been completed or are in progress.

Application of YMC products for peptide purification
Some of the world’s largest producers of peptides rely on YMC phases for analysis and purification of their peptide products. With one of the industry’s broadest product offerings for chromatographic separations in the field of peptides, YMC has a reputation for being the “go-to” source for phases, columns, lab services, and lab and GMP-scale HPLC systems. From our innovative YMC-Triart Hybrid Silica C18 chemistries to the disruptive Contichrom MCSGP technology, YMC is enabling peptide scientists to bring new products to market faster—with higher yields and purity. YMC’s chromatography solutions are complemented by our TFF, solvent blending, C&D, biosynthesis, and other process skids.
MCSGP: a game-changing innovation
Our patented Multicolumn Countercurrent Solvent Gradient Purification (MCSGP) technology is a game-changing innovation in the production of peptide-based drugs.
High performance liquid chromatography (HPLC) employing MCSGP provides a novel purification technology for peptides produced by chemical synthesis. MCSGP offers a step change in efficiency compared to batch HPLC processing. With MCSGP, two identical reversed-phase columns are operated under high pressures in countercurrent mode with internal recycling of impurity-containing side fractions, extracting continuously pure product and discarding impurities without significant product loss. This enables peptides to be purified at preparative/production scale with significantly higher yield—without compromising target purity. The process also allows up to a tenfold increase in productivity with typically 75% lower solvent consumption. This attractive economical production scenario sets a new standard for the economical synthesis of long peptides.
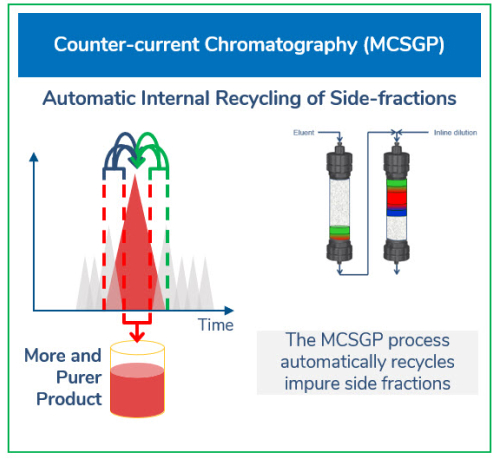
MCSGP process principle and design
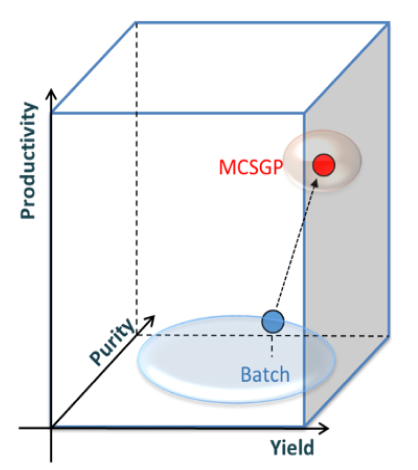
The MCSGP process is the only known ternary continuous separation process that can solve the yield-purity trade-off dilemma by providing high yield at target purity, thereby enhancing productivity.
The process eliminates the need for re-chromatography and the associated time and resources for sampling and testing. The patented MCSGP technology offers a process principle that is intuitive, straightforward, and allows a high degree of automation. This is visualized in an animation of the two-column MCSGP design. A dedicated software wizard in the accompanying ChromIQ software allows for easy batch-to-continuous process design, transfer, and optimization. ChromaCon offers customized services and expertise to companies and academics who wish to quickly advance their projects or test MCSGP capabilities before investing in a system.
Figure: Yield-purity-productivity relationship of MCSGP versus conventional batch chromatography. Single-column batch processes (blue) operate on a low productivity level without the potential for productivity gains when high yield and purity are required. MCSGP (red) operates at an optimum in 3D space, with high yield and purity at increased productivity.
Application Search
Use our extensive Applications Search page to mine a dynamically growing collection of over 350 YMC-produced application notes.
The data can be searched by any combination of several variables, including sample classification, compound name (including partial names), and column parameters.
