Monoclonal Antibodies (mAbs)
Advancing mAb Purification
Fundamental to the biopharmaceutical industry, the manufacture of therapeutic monoclonal antibodies (mAbs) has steadily advanced since their introduction into the market in 1986. As proteins with a highly defined structure produced from modified mammalian cells, mAbs can be engineered to have uniquely impactful therapeutic effects.
As demand for mABs has grown, so have calls for cost reduction. YMC has responded to this need by offering unique and innovative twin-column (continuous) technology that not only reduces the cost of mAbs, but also lowers the environmental impact and increases productivity.
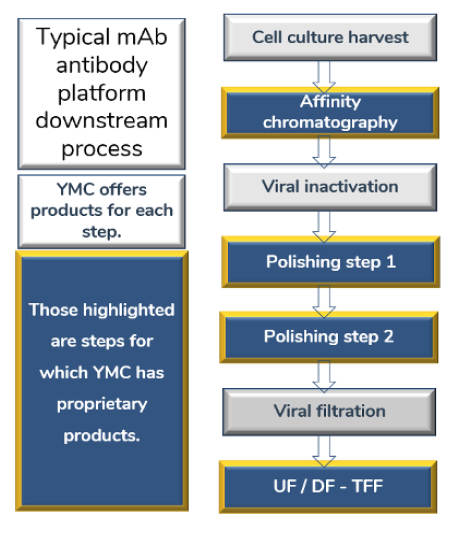
YMC offers an array of ways to enhance mAb-related separations.
Some examples:
- YMC’s Contichrom TWIN CaptureSMB systems drive down use of Protein A and associated buffers by up to 60%
- Our twin-column chromatography units increase productivity in mAb purification two- to three-fold
- YMC-Triart Bio C4 can be used for reversed-phase separations of intact mAbs and other large proteins
- YMC BioPro HIC BF is a new phase ideal for separation of antibody-drug conjugates by hydrophobic interaction (HIC)
- YMC BioPro Ion Exchange columns are useful for mAb charge variant analysis
- N-Rich® process amplifies impurities in mAb feed solutions, enabling better identification and quality control
- YMC ECO series glass columns can be used in all chromatography steps
Application Search
Use our extensive Applications Search page to mine a dynamically growing collection of over 350 YMC-produced application notes.
The data can be searched by any combination of several variables, including sample classification, compound name (including partial names), and column parameters.
